Home > Our Services > SecOps >
Security Risk Assessment for Life Sciences
From Precision Analysis to Actionable Insights
Security Risk Assessment for Life Sciences
Strengthen your security posture and compliance with a comprehensive Security Risk Assessment tailored for biotech, CRO, and life sciences organizations. Identify vulnerabilities, benchmark controls, and prepare for audits with expert cybersecurity guidance.
PTP’s assessment aligns with HIPAA, GxP, and FDA 21 CFR Part 11 frameworks, delivering practical remediation plans and actionable insight into your AWS and hybrid IT environments.
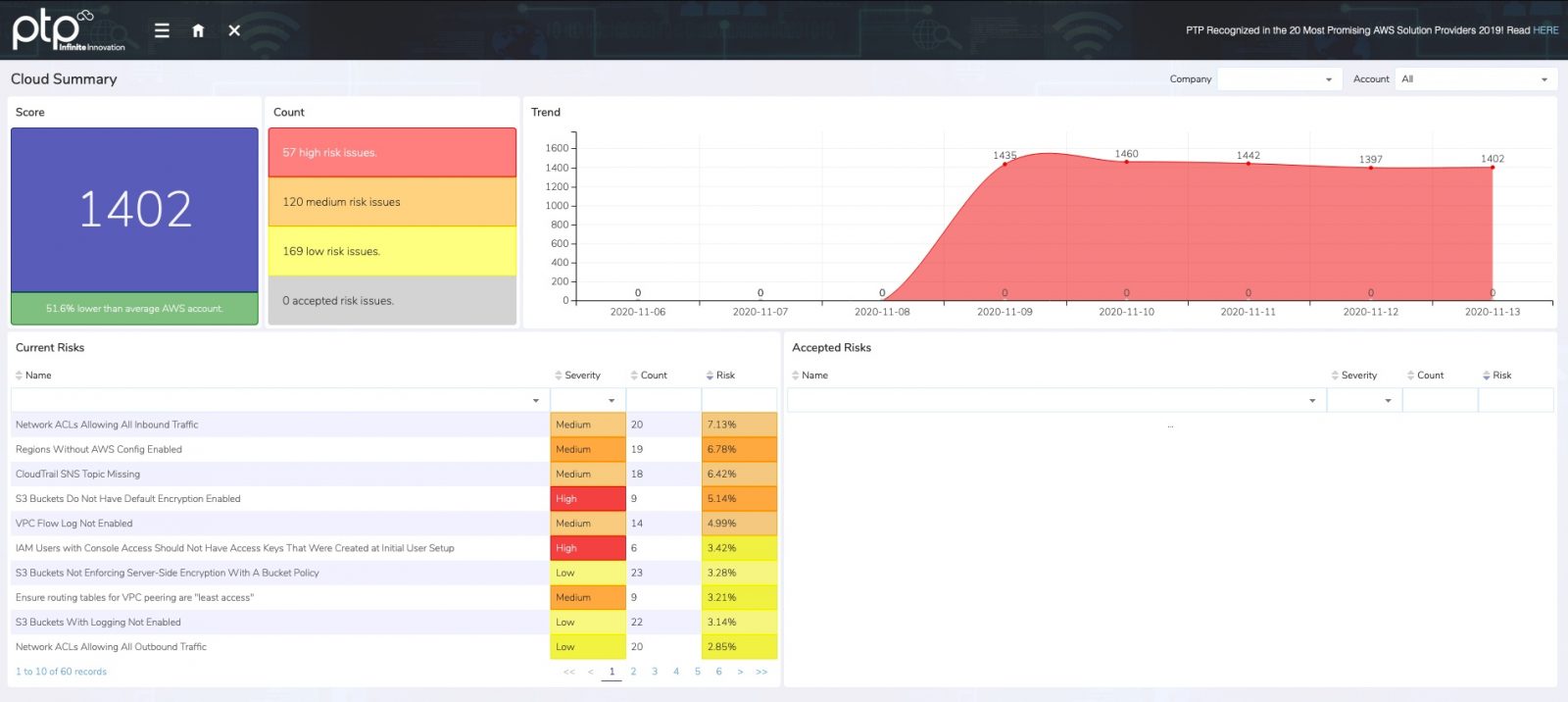
Assessment Output Sample
View a sample Security Risk Assessment report to see how PTP delivers clear findings, remediation steps, and compliance insights.
View Sample ReportWhat you gain
Clear findings
Severity-ranked insights that make it easy to understand and address your highest risks.
Prioritized remediation
Actionable recommendations organized by urgency for quick wins and long-term improvements.
Compliance alignment
Mapped controls that align with HIPAA, GxP, and FDA 21 CFR Part 11 compliance requirements.
Executive summary
Concise reporting tailored for leadership—ideal for audits, insurance, and board review.
What we assess
Device configurations
Configuration review to uncover vulnerabilities and misconfigurations.
Software and ports
Exposure checks for services, versions, and open ports that raise risk.
Network behavior
Traffic patterns by geolocation to surface anomalies and suspicious flows.
Threat scoring
Risk scoring based on indicators of compromise to focus effort where it matters.
The result is a concise report that lets teams review, prioritize, and act on immediate remediation while planning long term improvements to security posture.
Security Assessment Resources
Get 50% off Security Risk Assessment
Start mitigating vulnerabilities in your environment